Advantage
Experience: The department's primary registration specialists in average have more than 10 years of experience in regulatory affairs & registration for medical devices. Familiar with national policies and regulations, application procedures and relevant regulatory agencies. An excellent regulatory affairs & registration team can significantly reduce registration time costs and increase efficiency.
Team: Active Department, Passive Department, In Vitro Diagnostic Reagent (IVD) Department
Service Scope: For more than ten years, we have been dedicated to NMPA application of imported and domestically-made medical device products, as well as NMPA regulations training and consulting, etc. The company has successfully obtained registration certificates for more than 2,000 products. Extensive testing center resources and cooperation experience, familiar with application for innovative devices from NMPA and regulatory requirements and procedures for priority review, rich experience in application for medical device clinical evaluation report (CER) and success cases.
Service Scope
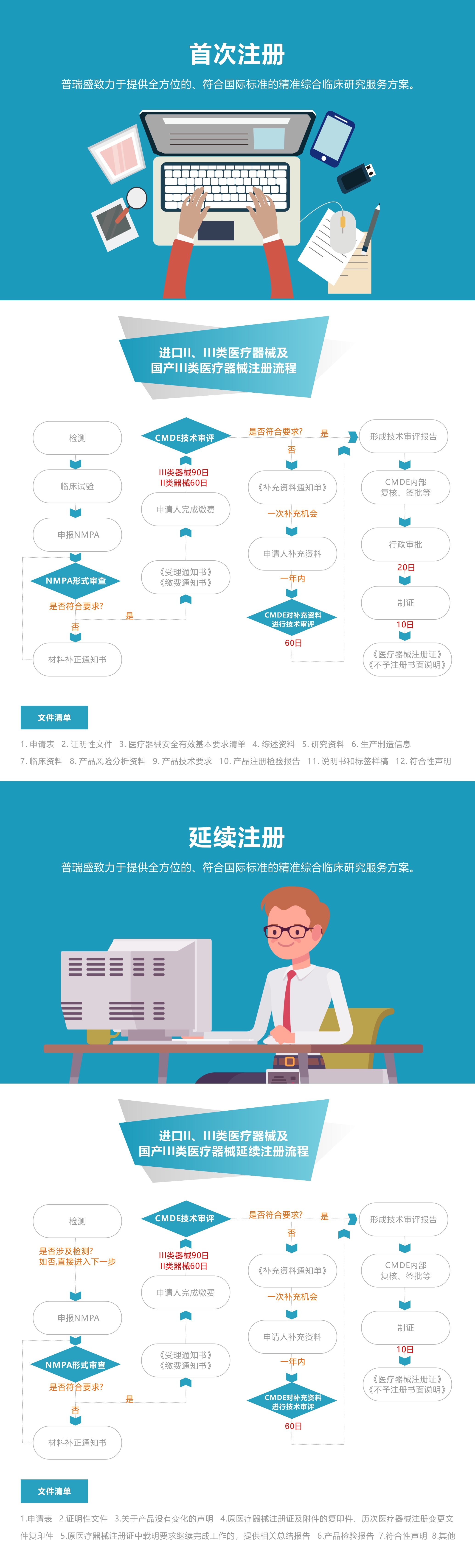
Technical requirements preparation
Assist in testing
Protocol design
Clinical report review
Application documents preparation
Submit application to NMPA
Expert review conference
Preparation of required supplementary documents
Tracking progress and taking certificate
Major project experience
Active
Medical electronic devices
Medical endoscope device
Medical ultrasound devices
Medical laser devices
Medical high frequency devices
Physical therapy devices
Medical magnetic resonance devices
Medical X-ray devices
Passive
Medical electronic devices
Medical endoscope device
Medical ultrasound devices
Medical laser devices
Medical high frequency devices
Physical therapy devices
Medical magnetic resonance devices
IVD
PCR
ELISA
Particle-enhanced turbidimetric immunoassay
Electrochemistry
Biochemistry
Tumor marker
Other
Software
Other